What is a biofilm?
Biofilms occur when a group of microorganisms (typically bacteria, fungi, parasites and viruses) attach themselves to a surface to create a colony. This colony form themselves into a type of ‘shield’ which has a glue-like or slimy consistency (Figure 1). Biofilms act as a barrier to the wound and help the colony to defend itself against antimicrobial treatments and immune cells (Curtis, 2020). This may be the reason that some wounds can be difficult to heal, why persistent infections may keep recurring and why antimicrobial resistance may be seen in some wounds in veterinary practice.
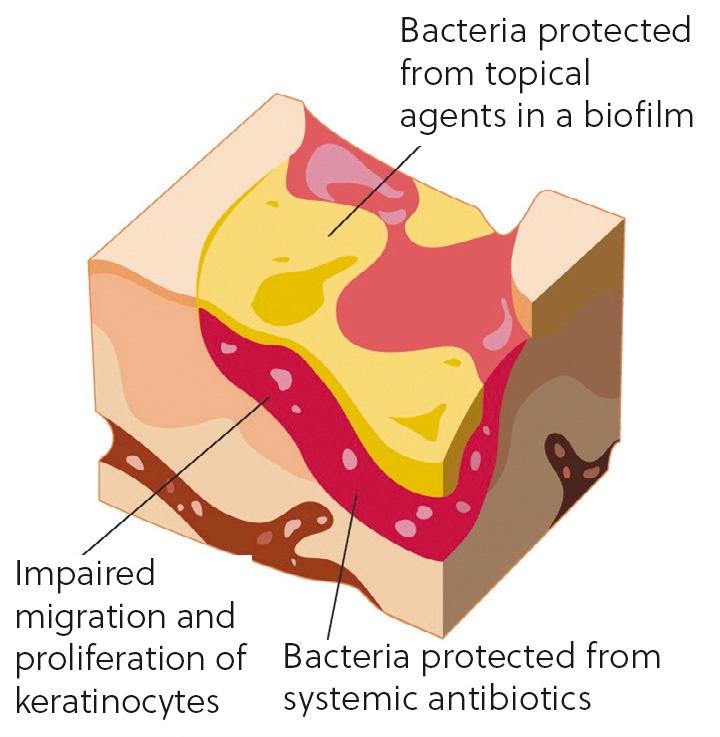
Antimicrobial resistance is a key concern in veterinary practice, via the One Health initiative, and refers to the ability of any microbes to resist treatment to drugs designed to kill them or stop their growth (HM Government, 2023).
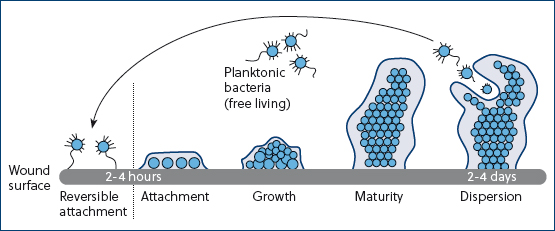
Free-floating microbes attach to the wound almost immediately and within 2–4 hours the bacteria have formed micro-colonies that securely attach to the wound (Curtis, 2020). The colony grows and matures between 2 and 4 days later (Figure 2). From the mature biofilm, bacteria can then disperse and attach to another area of the wound surface. This continues until the whole surface of the wound is covered with biofilm. This attachment can be reversible; however, it will require specific treatment. The colony secretes material that provides a structural matrix, similar to cement. These structures can adhere to surfaces and on medical materials, which can be the cause of hospital-acquired infections (Phillips et al, 2010).
But biofilms are not always undesirable. In areas such as our digestive system, for example, we all need healthy bacteria biofilms (Georgiou, 2017).
Assessment of wounds
It is useful to be able to describe wounds with accuracy to ensure that they can be dealt with efficiently. The aetiology of the wound is a factor that can prevent healing, so we can use this to help us describe wounds and to help with treatment planning. Knowing whether a wound is per-acute, acute or chronic can also help us plan our treatment.
We can further describe wounds by discussing the appearance of the wound bed tissues, which often involves a description of whether there is any granulation tissue or what its appearance is like. We can use the animal's presenting symptoms to help us. For example, a highly exudative wound is likely to be a chronic wound process and potentially the patient may look unkempt or have matted hair around the wound. We can also describe the wound by method of closure, for example whether it is a fairly clean, open wound that may be able to be closed immediately with sutures (first intention closure), or whether second intention closure is required, such as open wound management with dressings and bandages. Anatomical location is also very important, as wounds can stretch, spread and, of course, close, so describing location is important when assessing a wound long term.
A knowledge of wound classifications is essential to be able to triage and establish an initial plan for our patients. Categories include surgical, lacerations, abrasions, avulsion, de-gloving, shearing, puncture, burns, pressure sores and casts/bandaging injuries.
We also need to consider patient factors. Patients with wounds require immediate physical assessment of all body systems, alongside a detailed patient history. We may need to proceed with placement of an intravenous catheter and intravenous fluids to help stabilise the patient. Depending on the severity of the patient's presenting signs, we may then need to establish a neurological status, obtain some patient parameters, take blood profiles for analysis and administer analgesics. Using pain scores is a very useful way to establish what level of analgesia is required, and this should be repeated as necessary.
Only once we have assessed the body systems and ensured that we are happy with the patient's condition, can we then start think about the wounds presented. We then can start to control active haemorrhage and obtain samples, such as swabs, for culture and sensitivity if indicated.
Appropriate wound cleansing
Lavage is one of the most important parts of wound management, and there are multiple reasons why it is beneficial and will help to accelerate wound healing (Aldridge, 2013).
Lavage firstly rehydrates any potential necrotic tissue (Kirk, 2014), which can either help potentially to preserve some surrounding tissue and aid removal of any tissue not required by debridement. It will reduce bacterial contamination if conducted appropriately (Aldridge, 2013). Lavage will also reduce foreign material, if it is done correctly. The process will remove any toxins and cytokines from the wound and remove any surface debris and bacteria (Anderson, 2012).
Lavage can also help remove any remnants of wound dressings with minimal trauma to surrounding fragile tissue. It should be carried out at every dressing change, not just on the first presentation of the wound. The fluids used should be isotonic and non-toxic to cells. The main key principle is to ensure large volumes are used, so that even if all contaminants are not totally removed, they are at least diluted (Anderson, 2012).
Previously, lavage solutions contained antiseptics; however, they are now rendered obsolete for use within the wound itself. Other previous lavage solutions of povidone–iodine or chlorhexadine can also be harmful or even cell toxic in some cases (Hollis, 2018). It is also unlikely that any antiseptic would be in contact with the wound for an adequate amount of time to be effective. Tap water has been documented to be used to flush wounds, but it is hypotonic and therefore too alkaline for body tissue, so it should be avoided. Isotonic solutions are the best choice as they are non-toxic and will not cause cell rupture or electrolyte imbalance (Bell, 2021).
As isotonic solutions are the best choice, there are several options available. Hartmann’s is slightly superior to saline because of its buffering ability. TrisEDTA is an isotonic solution that has some evidence of useful antibacterial effects by enlarging pore size in antibiotic-resistant Gramnegative bacteria, therefore increasing bacterial kill rates (Anderson, 2012). The final product that can be used is Prontosan, which is a wound flush that contains a surfactant to break down bacterial biofilms.
To establish an effective first stage wound lavage, the correct equipment should be available. Large fluid volumes, such as 50 ml per centimeter of wound length, are essential for wound management. Using a giving set system can help with these large volumes. Using the following items: lavage solution, a three-way tap, an inexpensive sterile giving set, a 20 ml syringe and an 18 g needle, will effectively irrigate most wounds thoroughly (Figure 3).
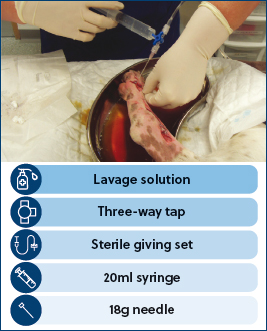
The pressures that should be delivered into a wound for lavage are still controversial. If we lavage at high pressures this will dislodge surface debris, but may also push this debris and bacteria further into the deeper tissue planes, which is detrimental (Bell, 2021). If we lavage at low pressures this may not be sufficient to remove debris from the wound. It is suggested that the pressure should be 6–8 psi, which is normally achieved by using a 20–30 ml syringe with 18 g needle attached. It will require a firm press on the plunger to create a steady stream to enable the correct psi to be achieved. Lavage should not be painful.
Prontosan can be used as a second stage lavage to help remove and prevent biofilms. It contains betaine, which is a gentle effective surfactant to penetrate, clean and remove wound debris and biofilm. It also contains polyhexanide (PHMB) which is an antimicrobial agent that can reduce bioburden in wounds. It cleanses, decontaminates and rinses acute and chronic skin wounds (Figure 4 and 5). The product can be used from first presentation of wounds in practice and continue to be used throughout the healing process until the skin is completely intact. This can prevent biofilm reforming.
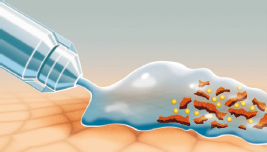

Prontosan can also be used to moisten dressings to facilitate gentle dressing replacements. Most importantly, it removes and prevents the formation of biofilm. The product requires 15 minutes of contact time with the wound bed and tissues. It can be used to soak swabs for packing into deficits and it also comes in a gel to place longer term in wounds to be then covered by a dressing. Prontosan gel helps to maintain a moist wound environment and provides a barrier to help prevent recontamination. The gel contains same ingredients as the solution but has a different consistency.
Prontosan is also widely used to gently irrigate wounds between dressing changes to help reduce or prevent biofilm formation in open wound management. Owing to its composition, it does not affect cells at the wound bed or edges and therefore will not degrade the fragile granulation tissue formation.
In conjunction with Prontosan, a Prontosan debridement pad can also be used. This can aid in debridement of devitalised and necrotic tissue, along with debris to help accelerate wound healing and reduce infection. By using the debridement pad in circular or vertical motions, the physical motion across the wound surface lifts any debris. There is valuable cleansing and debridement due to microfiber technology within the pad. The debridement pad is soft to ensure no further tissue irritation. The unique droplet shape of the pad allows debridement of cavities and areas that are difficult to reach. The pad is in a convenient blister packaging to allow easy aseptic soaking of the pad in Prontosan before use.
Conclusions
In summary, the use of lavage can reduce, remove and prevent biofilm formation in patients. Even if a biofilm is not present in a wound, using effective lavage techniques and products and using a product for biofilm prevention can help to reduce healing times in patients, and aid with the return to normal function quickly and more cost effectively. Lavage can be carried out by the veterinary team and is required throughout management of a wound, and not only at first presentation.
KEY POINTS
- Biofilms increase healing times, which can be detrimental to our patients.
- Removal of biofilms will reduce cost to our clients in the long term.
- Prontosan can be used from the first presentation throughout the healing process to help reduce and remove biofilm.
- In conjunction with a debridement pad, Prontosan can be used to reduce macroscopic debris to reduce the formation of biofilms and necrotic tissue formation.


