The domestic guinea pig (Cavia porcellus) is a popular children's pet and is also frequently used in laboratory animal medicine. As prey animals, they have evolved to hide their pain as a survival mechanism. Consequently, pain recognition for is challenging for both the pet owner and veterinary professional. This case study looks at a guinea pig who responded to chronic back pain with severe compulsive self-injurious behaviour. This resulted in a large, full thickness skin wound which was managed with a combination of support dressings, multimodal analgesia and manual acupuncture. Following wound healing, the underlying back pain was managed long term with non-steroidal anti-inflammatory drugs (NSAIDs) and electroacupuncture.
Clinician pain recognition and pain management in guinea pigs
Manifestations of pain in guinea pigs may be subtle, infrequent and inconsistent. Because they are a prey species, signs may be suppressed around humans as a survival mechanism (Turner et al, 2019). Accurate pain assessment is essential for maintaining animal welfare. Frustratingly, analgesia in guinea pigs ‘remains an empirical exercise based on anecdote, experience and best practice’ (Institute for Laboratory Animal Research, 2009), highlighting a critical need for evidence-based recommendations. Extrapolation from pain assessment tools used in rabbits and rodents is unreliable (Cohen and Beths, 2020).
Research into assessment of pain in guinea pigs has focused on three areas:
- Evoked responses
- Non-evoked responses
- Changes in physiological parameters (Turner et al, 2019).
These areas all pose difficulties in reliably and accurately assessing and measuring pain in guinea pigs and have their limitations (Table 1). Studies have focused on postoperative pain, which may manifest differently to chronic or neuropathic pain. Chronic pain has emotional knock-on effects, which the author speculates will result in chronic stress and associated physiological effects. Therefore, management is important for both physical and mental health. In the author's experience, bodyweight is correlated to ongoing illness, which could be considered an indicator of chronic pain, given that an increase in weight is seen following introduction of appropriate analgesia. Pain is a disease symptom, not a diagnosis, so underlying conditions must be managed. Analgesia should be instigated similarly in companion small animal species, using data from pharmacokinetic and pharmacodynamic studies where possible, to accommodate variation in drug metabolism (Swisher and Lennox, 2015).
Table 1. The three areas of assessment and measurement of pain in guinea pigs and their limitations
| Type of assessment and measurement of pain | Example | Difficulties in reliability |
|---|---|---|
| Evoked responses |
|
|
| Non-evoked responses |
|
|
| Changes in physiological parameters |
|
|
Adapted from Ellen et al (2016), Oliver et al (2017), Turner et al (2019)
The potential causes of underlying back pain in guinea pigs
Given the challenges in identifying pain in guinea pigs, spinal pain is likely oft en left undiagnosed. Deficiencies in both vitamin C and D can lead to skeletal abnormalities and pain in guinea pigs. Hypovitaminosis C (scurvy), a multisystemic disease, can present with reluctance to move, screaming on restraint and joints swelling (Clarke et al, 1980). Hypovitaminosis D, more common in satin breeds, can present with reluctance to walk or move and pathological fractures (Hawkins and Bishop, 2012). In addition, there is no anatomical reason that conditions resulting in chronic back pain in dogs (Table 2) could not occur in this species.
Table 2. Conditions which may present with chronic back pain in small animals using a modified degenerative, anomalous, metabolic, neoplastic, nutritional, inflammatory, idiopathic, toxic, traumatic and vascular model*
| Mechanism of disease | Specific examples that might present with chronic back pain |
|---|---|
| Degenerative | Cervical stenotic myelopathy, degenerative myelopathy, degenerative lumbosacral stenosis, dural ossification, foraminal stenosis, intervertebral disc disease, muscular dystrophy, peripheral neuropathy, spinal synovial cysts, spondylosis deformans, osteoporosis |
| Anomalous | Atlantoaxial instability, dysmyelination/hypomyelination, osteochondromatosis, scoliosis, vertebral and spinal cord anomalies |
| Metabolic | Calcium abnormalities, metabolic myopathies. |
| Neoplastic | Chordomas, nerve sheath tumours, paraneoplastic neuropathy, spinal neuroepithelioma, vertebral body tumours, vertebral plasma cell tumours |
| Nutritional | Hypovitaminosis D, hypovitaminosis C, Hypervitaminosis A, thiamine deficiency |
| Inflammatory (immune mediated and infectious) | Chronic inflammatory demyelinating polyneuropathy, discospondylitis, ganglioradiculitis, inflammatory spinal cord diseases, osteomyelitis, polyarthritis, spinal empyema, vertebral physitis |
| Idiopathic | Dysautonomia, disseminated idiopathic skeletal hyperostosis |
| Toxic | N/A |
| Traumatic | Caudal lumbar/lumbosacral trauma, cervical vertebral fractures and luxations, fractures and luxations of the caudal lumbar and sacral vertebrae, fractures and luxations of the thoracolumbar spine, lumbosacral plexus trauma, sacral fractures, sacrococcygeal fracture/luxation and tail avulsions, spinal cord contusion, thoracolumbar fractures and luxations, traumatic disc herniation |
| Vascular | Coccydynia, spinal haemorrhage |
Adapted from Platt and Olby (2014)
Many back-related pathologies result in neuropathic pain from stimulation of somatosensory nervous tissue in the central or peripheral nervous system. Humans report this as burning sensations in an area of paraesthesia (Platt and Freeman, 2014). Neuropathic pain is difficult to detect in animals and is inferred from patient behaviours, such as sudden movements or compulsive disorders (Lindley and Cummings, 2006). In humans, compulsive self-injurious behaviour targeted at underlying neuropathic pain has been reported (Mailis, 1996). Self-injurious behaviour is characterised by uncontrollable compulsion to traumatise painful body parts, undeterred by subsequent tissue damage and pain. Neuropathic pain is refractory to classical analgesics such as NSAIDs and opioids, and treatment involves anticonvulsant and antidepressant drugs (Smith et al, 2001). The guinea pig in this case presented with a compulsion to traumatise a specific anatomical area, suggesting underlying neuropathic pain.
Back injuries as a source of chronic pain
Guinea pigs are small pets at risk of traumatic misadventure. Injury to the following may result in chronic back pain (Platt and Freeman, 2014):
- The meninges covering spinal cord
- The spinal nerves/nerve roots of peripheral nerves
- The cartilaginous intervertebral disc and surrounding annulus
- The bony vertebrae, vertebral periosteum and articular joint capsule
- Surrounding epiaxial musculature.
Vertebral fractures or luxations may occur following falls, cage trauma, predator attacks or improper handling (Zehnder and Kapatkin, 2012). These manifest differently in different regions of the vertebral column because of varying anatomy. Vertebral fractures or luxations commonly occur at the thoracolumbar junction (T10–L2) because of its position between the rigid thoracic spine and well-muscled lumbar spine. Such injuries are often associated with traumatic intervertebral disc extrusion (Fenn and Olby, 2020), where internal contents of intervertebral disc herniate through surrounding annulus. Neurological deficits are not always present and subsequent degenerative changes and chronic pain may occur.
Chronic non-healing vertebral fractures have been reported causing chronic pain in guinea pigs (Zehnder and Kapatkin, 2012). In horses, fractures of dorsal spinous processes are reported to occur following traumatic events presenting with pain, swelling and reluctance to move associated anatomical areas (Henson, 2019). Fracture fragments can be displaced cranially, caudally or axially (Molnar et al, 2012). Prognosis depends on fracture number, fragment displacement and type of healing: comminuted displaced or unstable fractures suffer prolonged healing, whereas inadequate reduction and immobilisation leads to delayed bony or fibrous healing.
Vertebral luxations following trauma have been reported in guinea pigs (Hammons, 1979). Torsional force causes luxations often occurring concurrently with fractures of restraining processes (Jeffery, 2010). Vertebral fractures or luxations can cause neurological injury, resulting in pain from neural compression, direct mechanical trauma and instability of mesenchymal tissues and compression of peripheral nerves.
In small mammals, vertebral fractures or luxations are difficult to surgically manage because of their small size and relative lack of bone strength. Therefore conservative management is opted for, with rest and symptomatic treatment with or without external body support (Guzman and Kapatkin, 2021). Healing resulting in scoliosis may cause spinal discomfort and chronic pain via denervation and unilateral atrophy of epiaxial muscles and formation of myofascial trigger points (Platt and Freeman, 2014).
What is acupuncture and how does it help to manage pain?
Acupuncture is the insertion of a solid needle into the body for the purpose of therapy, disease prevention or maintenance of health (Acupuncture Regulatory Working Group, 2003). These needles are stimulated by hand (manual acupuncture) or electrical current (electroacupuncture).
There are three areas where acupuncture needles are placed:
- Acupuncture points: the name and number of which are derived from traditional acupuncture, but their use depends on anatomical and neurophysiological significance
- Myofascial trigger points: painful points in skeletal muscle, characterised by taut bands and pain referral
- Tender points: tender areas occurring in muscle, at muscle–tendon junction, joint bursa or fat pad.
Acupuncture is often associated with traditional Chinese medicine and has been practiced in the east for over 3000 years. Since the late 1900s, there has been a surge in research into the scientific mechanism by which acupuncture works. Subsequently, a western approach has been developed which focuses on using points which help to relieve pain and relax muscles (Lindley and Cummings, 2006). There is now a wealth of literature supporting the use of acupuncture for human pain management (British Medical Acupuncture Society, 2010). Increasingly, western veterinary acupuncture is being used in pain management in animals (Lindley and Cummings, 2006).
Acupuncture affects pain by:
- Local effects: increases local blood flow and stimulates release of local neurotransmitters involved in wound healing. Can also deactivate myofascial trigger points
- Segmental: stimulation of fast acute A-delta pain fibres. Competition with C-fibre pain at the dorsal horn reduces the chronic pain signal
- Heterosegmental: brainstem response releases β-endorphins, noradrenaline, serotonin and oxytocin and enhances the descending inhibitory pain response at every spinal segment
- General: release of endorphins, adrenocorticotropic hormone and oxytocin alter the patient's pain perception.
Acupuncture as an adjunct to wound healing
Acupuncture supports wound management by providing analgesia and local tissue affects. Use of analgesia to facilitate wound healing is well documented (Mickelson et al, 2016) and is important in guinea pigs, where pain can result in decreased food consumption, gastrointestinal ileus, weight loss, rapid decline and death. Acupuncture has been used to support healing of localised wounds by secondary intention (Lundeberg et al, 1988). Some mechanisms of action may include increased blood flow through release of neurotransmitters, such as substance P, calcitonin gene-related peptide and vasoactive intestinal peptide, as well as by reducing oedema and stimulating nerve growth factor and local nerve regrowth into wounds. Healing can be promoted by inserting needles into normal tissue encircling the wound periphery.
The use of acupuncture in back pain caused by vertebral fractures and luxations
Following veterbral fractures and luxations patients may experience chronic pain as result of delayed healing; non-unions; fragment displacement; degenerative changes adjacent to trauma site; disrupted spinal biomechanics caused by scoliosis formation resulting in chronic soft-tissue or arthritic pain (Kendler et al, 2016). Acupuncture may help alleviate this through local, segmental, heterosegmental and general effects via the stimulation of local acupuncture points along the back. Table 3 outlines acupuncture points commonly used for back pain in animals (Janssens, 2001; Schwart, 2001). Some variation in point position is seen between species based on anatomy and vertebral formula and have been extrapolated for use in the guinea pig in Figure 1a and 1b.
Table 3. The position of relevant acupuncture points for back pain
| Acupuncture point (cranial to caudal) | Position |
|---|---|
| GV 14 | In the midline, between spinous processes of most caudal cervical vertebrae and first thoracic vertebrae |
| BL 15 | Either side of the vertebral midline at the level of the fifth intercostal space into the paraspinal muscle |
| BL 17 | Either side of the vertebral midline at the level of the eight intercostal space into the paraspinal muscle |
| BL 19 | Either side of the vertebral midline at the level of the tenth intercostal space into the paraspinal muscle |
| BL 21 | Either side of the vertebral midline at the level of the twelfth intercostal space into the paraspinal muscle |
| BL 23 | Either side of the tip of transverse process of L2 |
| BL 25 | Either side of the tip of transverse process of L4 |
| GV 3 | Midline between spinous processes of most caudal lumbar vertebrae and first sacral vertebrae |
| BL 28 | At level of S2, just caudomedial to dorsal iliac spines, through multifidous muscle. Bilateral points. |
| GB 30 | Bilateral point located 2/3rds the distance from midline to pelvic wing, directed towards pubic symphysis through the most prominent point of the gluteal muscles |
| GB 34 | Lateral aspect of each stifle into the depression craniodistal to head of fibula through the peroneus longus muscle |
| ST 36 | Lateral leg just distal to each stifle. Half way down the tibial crest into the tibialis cranialis muscle |

There is uncertainty regarding the effectiveness of acupuncture in managing neuropathic pain (Ju et al, 2017). Chrisman (2014) summarised the use of acupuncture for controlling pain associated with intervertebral disc disease in dogs and found that, subsequent to acupuncture, doses of analgesics may be reduced. Acupuncture may have helped in this case by providing local comfort, relieving associated trigger points and managing associated chronic pain.
Case study
A 6-month-old, underweight, entire male guinea pig presented with a full skin thickness, non-healing wound on the dorsal thorax. The most extensive area was on the left-hand side and was 2 cm in length by 4 cm in width, extending into the underlying musculature. This progressed into a thinner laceration on the right-hand side of the body. The animal's back was allodynic on light palpation demonstrated by flinching, vocalisation, back arching and weight shifting. The owner had acquired the animal patient 1 month earlier, with no previous history, and the wound occurred shortly after arrival. The possibility of self-injurious behaviour was discussed, but the owner reported that the animal had been attacked by the bonded cage mate. The guinea pig was otherwise well and there were no neurological deficits. A microbiological swab was taken for culture and sensitivity, which resulted in no significant growth.
Over the subsequent 8 weeks, the wound was managed with support dressings, multimodal analgesia and manual acupuncture. The progression of wound healing can be seen in Figure 2. Sterilised disposable J-15 type acupuncture needles (0.16 mm×15 mm in size; Seirin, Japan) were used throughout acupuncture treatment and left in situ for approximately 5 minutes. GV 14 acupuncture point was used to assess the patient's reaction to needles. It was too painful to apply other needles cranial to the wound so a perisegmental (above and below the affected area) approach was used (Figure 3). Granuflex dressing (ConvaTec, USA) was then applied to aid autolytic debridement of necrotic sloughy tissue and to promote secondary wound healing (Figure 4). This was secured with Cohesive bandage (NVS, UK). The patient was prescribed Meloxicam (Metacam, Boehringer Ingleheim, UK) (empirical dose 1 mg/kg per os, twice daily) and seen for acupuncture and dressing changes weekly for 8 weeks until wound had fully healed.

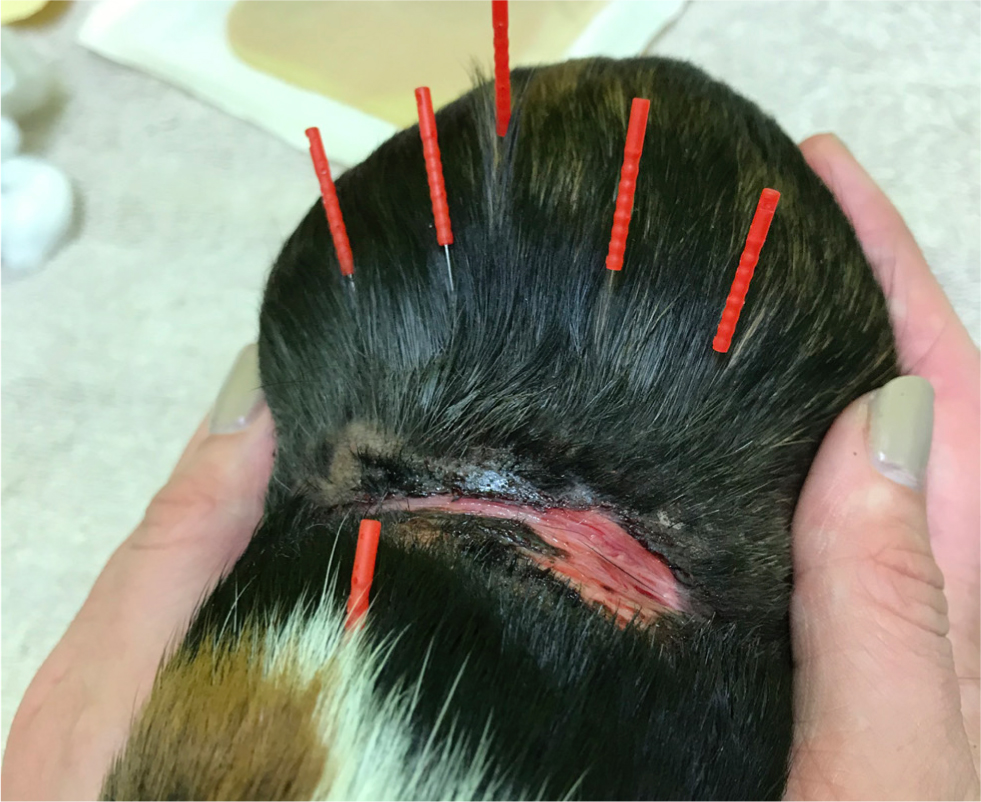

In week 8 the patient was admitted for removal dressings under observation and was viewed immediately scratching and biting himself. Radiographs were performed to investigate the underlying cause (Figure 5a and 5b) and underlying spinal pathology confirmed. The presenting wound was situated over T9 to T12. Pathological changes were present from T10 to T13. There was considerable soft tissue swelling and fractures to the dorsal spinous processes were suspected. A luxation of T13 was resulting in compression of T12/T13 disc space. On the ventral-dorsal view there was disc compression on right-hand side at T10/T11 and T11/T12 and on the left-hand side at TT12/T13 and T13/L1. Lumbar spine showed disruption to continuity of paraspinal muscles from L2 to L4 with an opacity obscuring dorsal spinous processes of L3 and L4 and variation in angulation between multiple lumbar vertebrae. More advanced imaging such as computed tomography would have been useful, but this was unavailable.

Following radiographic assessment additional analgesia was prescribed; Tramadol (MercuryPharma, UK) (10 mg/kg per os intramuscularly, twice daily) and Gabapentin (Milpharm limited, UK) (10 mg/kg per os, twice daily). Manual acupuncture was performed at GV14, BL17 and BL21, BL23 and BL25 and BL28 (see Figure 1b for their anatomical positions). The patient appeared comfortable and showed relaxed grooming behaviour during acupuncture (Figure 6). Body dressing was reapplied to prevent further self-injurious behaviour and to provide support for underlying spinal damage. The patient's environment was modified to encourage rest and prevent further exacerbation of the injury. A smaller ‘rest’ cage was used with no climbing furniture, multiple accessible hiding places were added, and the position and height of food and water sources was adjusted for easy access.
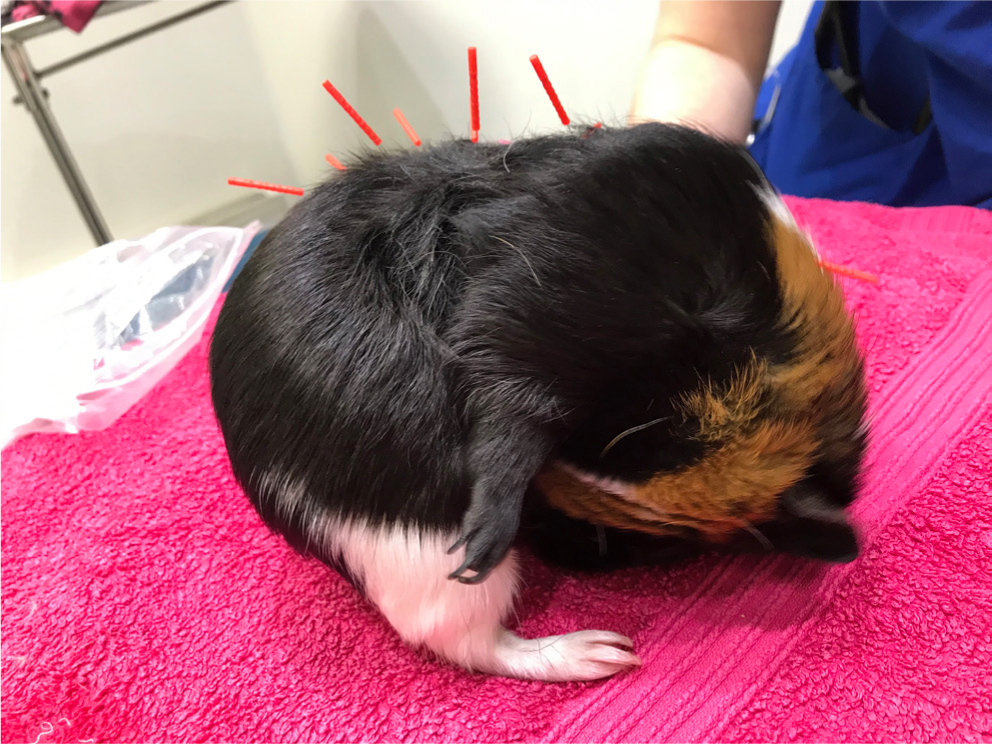
Manual acupuncture and body dressing was repeated at weeks 9–12. The patient subsequently presented in week 16 and the dressing was removed but self-injurious behaviour recurred. Amitriptyline (Bristol laboratories Ltd, UK) (5 mg/kg once daily) was additionally prescribed and dressing reapplied. Concerns were raised regarding long-term quality of life.
The patient next presented in week 20, when both the patient and its bonded cage mate were rehomed to a member of the veterinary team. The wound had completely healed and the decision was made to remove the dressing, stop amitriptyline and start 6 weeks of weekly electroacupuncture (Figure 7), alongside Meloxicam, tramadol, gabapentin and HEV'N anti-inflammatory mix (1 tablespoonful daily; Hop to forage, UK), a forage-based diet supplement containing plants which support a reduction in inflammation. No signs of self-injurious behaviour were seen after the third session of electroacupuncture, and the doses of tramadol and gabapentin were reduced and then completely withdrawn by the sixth session (week 26).
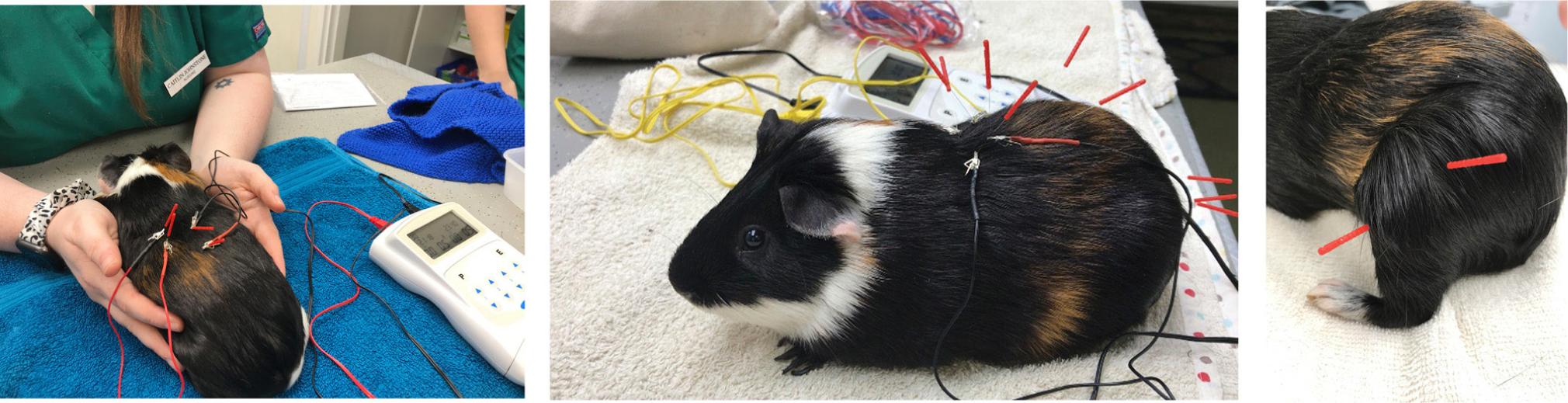
The electroacupuncture protocol was adapted and modified for a guinea pig from Lee et al (2005). Acupuncture points immediately cranial and caudal to damaged thoracic vertebrae were located; BL17 and BL21. These were stimulated using an electroacupuncture device (AS SUPER 4 Digital Needle Stimulator, Schwa Medico, Germany) set at dense dispersed setting of 2 Hz and 15 Hz. The mAmp was varied for patient comfort and ranged from 0.3 mAmps to 0.8 mAmps. Manual acupuncture was performed at points in the pelvic limbs: GB 34 and ST 36; and in the epaxials and over the sacrum (BL23, BL25 and BL28), because of the lumbar radiographic changes. All needles were left in situ for approximately 5 minutes. After 6 weekly electroacupuncture sessions the frequency was reduced to monthly sessions.
At week 34, repeated radiographs were taken to review ongoing spinal damage (Figure 8a and 8b). Owing to the significant inflammation reduction, visualisation of fractures of dorsal spinous processes at T10, T11 and T12 was enabled. Subluxation of T13 resulting in compression of T12/T13 disc space was still present. Despite ongoing spinal pathology, to date, there has been no return of self-injurious behaviour with continued management with Meloxicam, HEV'N anti-inflammatory mix and monthly electroacupuncture sessions (week 52).

Case discussion
Initially, the presenting wound was attributed to conspecific conflict and the owner reported no signs of pain, so found it difficult to consider wounds were self-inflicted, highlighting the common difficulties in recognising pain and understanding guinea pig behaviour. It is impossible to conclude whether acupuncture resulted in accelerated wound healing in this case, because the patient repeatedly removed dressings and re-traumatised the wound. In addition, it is not possible to predict how long healing without acupuncture would have taken. Open wounds in exotic small mammals are reported to take several days to months to heal by second intention (Mickelson et al, 2016) and no deviation from that was seen.
Bodyweight appears to be correlated with ongoing illness and chronic pain in guinea pigs, but this needs to be interpreted in a clinical context. At 6 months, the patient would be expected to gain weight as a result of growth. The body condition score was subjectively measured during clinical exams by palpating the muscle and fat coverage over the ribs, lumbar vertebrae and pelvic bones. It was measured using a 1–5 scale, with 1 being underweight with minimal coverage and 5 being overweight with bony prominences not palatable. There was a trend for both the patient's body condition score (Figure 10) and weight (Figure 11) to increase throughout the treatment, suggesting that the weight gains were not solely attributable to growth but to improvement of general condition, suggesting better disease management and analgesia.


Figure 11 shows the progression of the patient's bodyweight (grams) over the treatment duration of 34 weeks. Between weeks 1 and 8, bodyweight increased by 3% suggesting a small increase in comfort with weekly acupuncture and twice daily Meloxicam (NSAIDs and manual acupuncture have been reported to provide limited neuropathic pain analgesia so this approach was potentially limited). Following addition of gabapentin and tramadol, there is an increase in bodyweight of 17%. Although there is no literature to support their use in guinea pigs (Carpenter and Marion, 2018), this may suggest a significant analgesic effect.
In week 20, electroacupuncture was incorporated into the treatment regime. Since the patient was previously acclimatised to manual acupuncture before starting electroacupuncture this was accepted well. During electroacupuncture the needles are stimulated by an electric current: this appears to produce a greater range of neurotransmitters which may enhance the effects of manual acupuncture. It is often used in cases where pain control is considered more challenging and it is generally regarded to provide ‘stronger’, longer lasting effects (Lindley and Cummings, 2006). For these reasons its use was incorporated at needle positions close to the areas of thoracic spinal injury. Additional analgesia was then slowly withdrawn, bodyweight and body condition score remained stable and self-injurious behaviour did not return. This suggests that subsequent to receiving other pain medications, weekly electroacupuncture and meloxicam was maintaining analgesia sufficiently, or that the pain had been wound down by the other medications. After six weekly electroacupuncture sessions, the frequency of sessions was reduced to monthly. The effects appeared to persist and the patient's condition did not deteriorate.
The patient's self-injurious behaviour was suggestive of neuropathic pain which can be managed with tricyclic antidepressants. No guinea pig dose of amitriptyline is reported and so a dose of 5mg/kg daily was extrapolated from those reported in the rat (Carpenter and Marion, 2018). In humans, drug interactions have been reported between tramadol and amitriptyline, resulting in serotonin syndrome (Kitson and Carr, 2005). The pharmacokinetics of this combination in guinea pigs is not known, so the owner was instructed to monitor closely and no ill effects were reported. Treatment with Maropitant may also be considered in similar cases to this. In mice models, through blocking substance P binding to NK-1R in primary sensory neurons of spinal cord dorsal roots, maropitant (1 mg/kg) appears to reduce pruritis and stop the inflammatory cascade that results in self-injurious behaviour (Williams-Fritze et al, 2011; Williams et al, 2012; Di Mattia et al, 2017).
One argument often proposed against the efficacy of acupuncture is that pain decreases over time because of healing. However, while repeated radiographs showed improvement, significant pathological changes were still present and were likely to still be causing underlying pain. To demonstrate that the patient was still experiencing back pain from these injuries and that electroacupuncture had been maintaining analgesia, electroacupuncture would need to have been withdrawn and patient's condition monitored. However, withdrawing treatment on these grounds would be unethical and a welfare concern. Biannual radiographs and weekly weight checks will be continually be performed to monitor the patient long-term.
Conclusions
It can be challenging to recognise pain in guinea pigs and self-injurious behaviour can be considered a possible indicator of underlying chronic or neuropathic pain. The patient's self-inflicted wound was successfully managed with a combination of body bandages, multimodal analgesia and acupuncture but it is impossible to conclude whether acupuncture accelerated wound healing. Amitriptyline stopped the cycle of self-injury and no ill effects were seen with concurrent tramadol use. Patient bodyweight, alongside body condition score, can be a good indicator of pain although it can be non-specific. Following multimodal analgesia, patient weight and body condition score increased in this case. Once electroacupuncture was initiated the patient was able to be weaned off all other treatment for chronic back pain except NSAIDs and HEV'N anti-inflammatory mix. This combination appeared to provide adequate analgesia and the patient's condition remained stable.
KEY POINTS
- It can be challenging to recognise pain in prey species and when self-injurious behaviour is present, this could be a possible indicator of underlying chronic or neuropathic pain.
- In guinea pigs, clinical examination and cage-side evaluation alone may not provide a reliable indicator of patient pain and a multi-assessment approach including evoked, non-evoked and clinical measurements should be used.
- Patient bodyweight, along with body condition score, can be a good indicator of ongoing illness and suggestive of chronic pain.
- Amitriptyline could be considered to help break the cycle of self-injurious behaviour in guinea pigs. In this case, it was used alongside a multimodal analgesia approach with tramadol, gabapentin and meloxicam with no ill effects.
- The use of acupuncture and electroacupuncture could be considered for long-term management of chronic pain in guinea pigs, alongside non-steroidal anti-inflammatories and HEV'N anti-inflammatory mix.

