Long bone fractures in small animal patients such as rodents, rabbits and ferrets are frequently the result of traumatic events such as being trapped in wire cages and wheels or being stepped on or dropped by the owner (Miwa and Carrasco, 2019). Femoral fractures account for 30-40% of rabbit long-bone fractures (Sasai et al, 2018; Garcia-Pertierra et al, 2019). These fractures often occur in rabbits less than 2 years of age and are thought to result from the herbivores’ relatively thin and light cortical bone composition (Miwa and Carrasco, 2019).
In rabbits, common locations for femoral fractures include the condyle, distal and mid diaphysis (Garcia-Pertierra et al, 2019; Dorlis et al, 2022). Despite the distal femur being the most frequent site of physeal fracture in canine patients (Sukhiani and Holmberg, 1997), naturally occurring distal femoral physeal fractures in rabbits do not feature within currently published case series’. The only case report detailing naturally occurring femoral physeal fractures reports spontaneous capital physeal fractures in a Continental Giant rabbit (Knudsen and Langley-Hobbs, 2010).
Rabbits are commonly used as an animal model for experimental research as a result of considerations regarding economics, animal handling and ease of achieving ethical approval (Mapara et al, 2012). While histologically, rabbit long bones have a very different microstructure from the bones of human beings (Wang et al, 1998; Martiniakova et al, 2005), some similarities exist between mid-di-aphyseal bone mineral density and fracture toughness (Wang et al, 1998). The use of rabbits as an animal model results in an abundance of literature covering experimentally induced fractures, but extrapolating this to the clinical situation is not straightforward. Bias is a well recognised cause of poor concordance between preclinical and clinical outcomes with animal study design playing an important role in this (Ioannidis, 2012). At best, experimentally induced fractures may be considered an approximate model for traumatically induced fractures; a standardised surgical approach followed by drilling a hole across a physis for example (Antero Makela et al, 1988) will not accurately replicate a traumatic injury to either the physis or the surrounding soft tissues.
Comparison of femoral fracture fixation methods in rabbits is complicated by the paucity of studies detailing clinical patients, extensive sample variability and relatively small sample sizes. Stabilisation methods reported vary depending on fracture configuration but include the use of positional or lag screws, external skeletal fixators with or without an intramedullary pin, or bone plates placed in bridging or neutralisation mode (Sasai et al, 2018; Garcia-Pertierra et al, 2019; Dorlis et al, 2022). High rates of postoperative complications have been reported in current case series, ranging between 40 and 50% (Garcia-Pertierra et al, 2019; Dorlis et al, 2022). Complications that have been described include development of delayed or non-union, implant failure, iatrogenic fractures, pressure ulcers and osteoarthritis (Garcia-Pertierra et al, 2019; Dorlis et al, 2022). Neither the frequency of complications nor the attainment of functional recovery has been shown to differ between methods of fixation.
Various fixation techniques have been described for the repair of distal femoral physeal fractures in dogs and cats, including the use of intramedullary pins (Shires and Hulse, 1980), cross-pins (Milton et al, 1980), paired convergent pins (Franczuski et al, 1986) and modified Rush pins (Whitney and Schrader, 1987). The cross-pin method is commonly used with the most effective method, which involves the insertion of normograde pins in the distal to proximal direction, beginning on the non-articular surfaces of the femoral condyles (Sukhiani and Holmberg, 1997). An experimental study investigating two stabilisation methods following distal femoral epiphyseal separation in growing rabbits demonstrated that cross-pin stabilisation with two Kirschner wires reliably achieved fracture healing and showed no evidence of growth retardation at 24 weeks after the surgery (Antero Makela, 1988).
To the authors’ knowledge, there are no clinical case reports detailing distal femoral physeal fracture stabilisation in rabbits. This report describes the presentation, treatment and medium-term follow up of a distal femoral physeal fracture in a miniature Rex rabbit.
Case report
A 5-month-old, female spayed, indoor miniature Rex rabbit weighing 1.4 kg was presented with right hindlimb lameness. On the day of presentation, while in a playpen, the rabbit was startled by a loud noise and became entangled in the net of the cage. While attempting to free itself, the rabbit's right hindlimb was injured. Severe lameness was noted afterwards. At presentation, instability affecting the distal right femur had been noted, and mediolateral non-sedated radiographs revealed a Salter-Harris type I fracture of the right distal femur. Radiographs were performed at this stage in order to communicate with the owner about prognosis and potential referral options. As radiographs would likely be repeated following referral and a single mediolateral view was sufficient for diagnosis, the inherent risks associated with sedation were not considered to be warranted at this time. The rabbit was hospitalised and given subcutaneous 0.5 mg/kg meloxicam once daily, oral metoclopramide 0.5 mg/kg three times daily, and subcutaneous buprenorphine 0.01 mg/kg three times daily, pending orthopaedic referral. No external coaptation was considered to be indicated. The animal had cage-rested.
At referral, 48 hours later, a general physical examination was unremarkable. Gait assessment revealed reluctance to move and a severe right hindlimb lameness with the limb abducted during standing and movement. Orthopaedic examination revealed instability, crepitus and soft tissue swelling associated with the distal femur. The remainder of the orthopaedic examination was unremarkable.
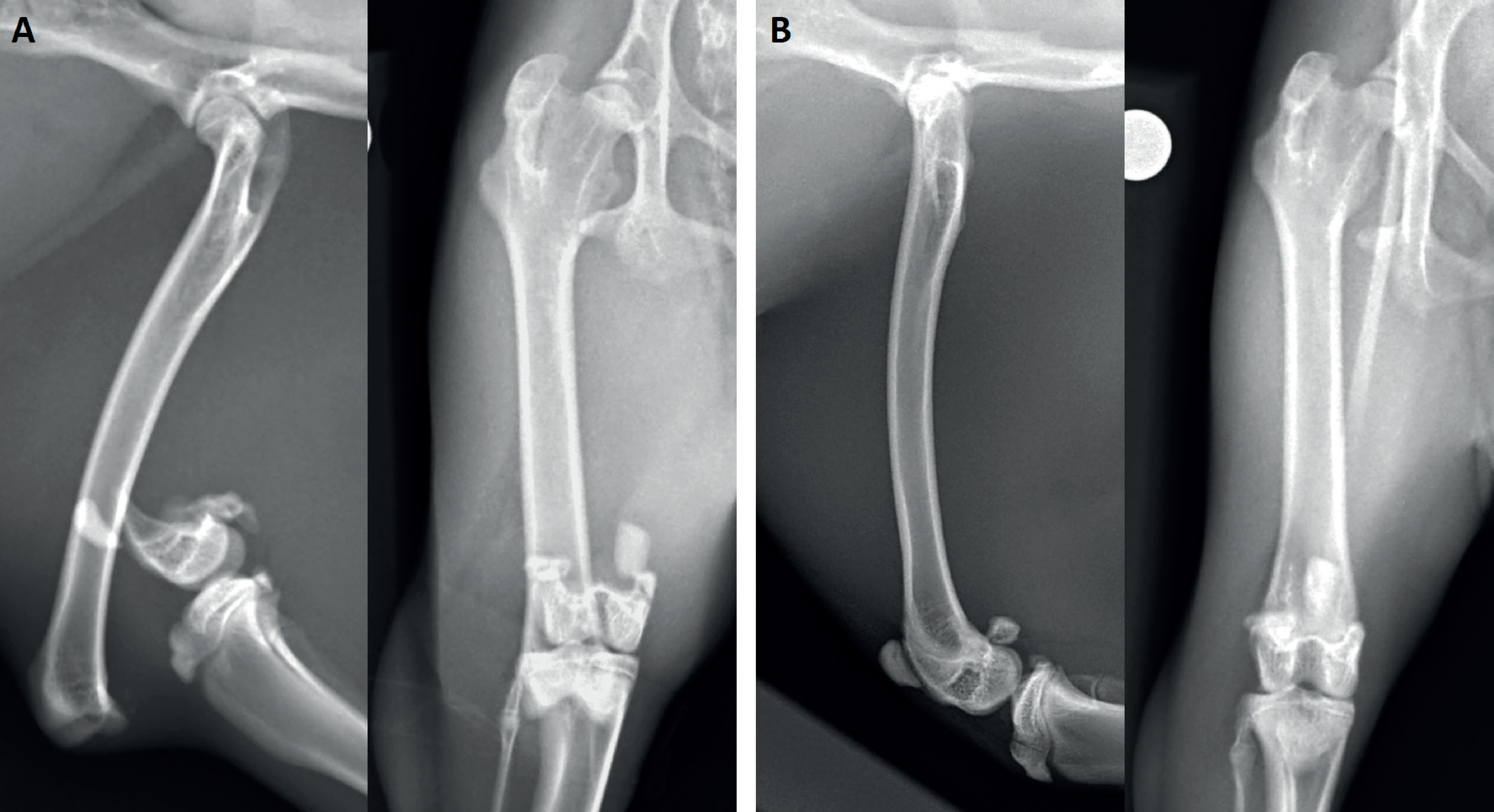
Accurately positioned craniocaudal and mediolateral femoral radiographs were required for preoperative planning. Food was withheld for 2 hours to reduce gut fill. Sedation was achieved using alfaxalone (2 mg/kg) and methadone (0.5 mg/kg) administered intramuscularly. Oxygen was delivered throughout via face mask. A heating pad and bubble packing were used to reduce heat loss. Electrocardiogram, pulse oximeter readings and the animal's temperature were monitored throughout. Radiographs (Figure 1) revealed a severely displaced Salter-Harris type I fracture of the distal femur on the right with moderate associated soft-tissue swelling. The left femur was within normal limits with physes remaining open as expected for a rabbit of that age. The patient recovered from sedation without incident. Metoclopramide and meloxicam were continued as detailed previously.
The following day, Eutectic Mixture of Local Anaesthestics (EMLA) cream was applied over the marginal ear vein for 1 hour which enabled pain-free intravenous catheter placement. Food was withheld for 2 hours. Premedication using dexmedetomidine (8 mcg/kg), ketamine (0.7 mg/kg), methadone (0.5 mg/kg), alfaxalone (2 mg/kg) intramuscularly was performed and induction achieved using intravenous administration of alfaxalone to effect. The rabbit was then allowed to breathe 100% oxygen via face mask for 3-4 minutes before intubation was attempted. Intubation was achieved using a 2.5 mm endotracheal tube and anaesthesia maintained using sevoflurane. Lactated Ringer's solution was administered at 7 ml/kg/hour throughout anaesthesia and perioperative antibiosis was provided using 5 mg/kg enrofloxacin administered intravenously. A circulating water blanket and bubble packing were used to reduce heat loss. With the patient in dorsal recumbency, the right hindlimb was clipped and surgically prepared using a hanging limb technique.
A craniolateral arthrotomy of the right stifle joint was combined with a lateral approach to the distal femur. Fragment-holding forceps were not used on the distal fragment to avoid articular cartilage damage and iatrogenic condylar fracture. Fracture reduction was achieved using ligamentotaxis via manipulation of the proximal tibia. The stifle was held in flexion with the tarsus in extension to relieve tension on the gastrocnemius muscle. Cranial traction was then applied to the proximal tibia, which positioned the femoral condyles cranially facilitating re-engagement of the metaphyseal pegs within the epiphyseal grooves. Following this, the fracture was intrinsically stable. The stifle was held in extension while stabilisation was achieved. Stabilisation was achieved using four 0.7 mm cross pins, two from medial to lateral and two from lateral to medial. Pins were placed normograde, from distal-to-proximal, with the initial insertion points on the non-articular surface of the femoral condyles just caudal to the medial and lateral aspects of the femoral trochlea. Intraoperative fluoroscopy was used to confirm appropriate implant placement with a cross-over point located above the fracture line. A 5 mg/kg dose of liposomal bupivacaine (Nocita) was instilled subcutaneously during closure. The remainder of closure was routine with intradermal 1.5 metric Monocryl Plus Antibacterial (poliglecaprone 25) suture (Ethicon) being used in the skin.
Postoperative radiographs (Figure 2) confirmed anatomical fracture reduction, limb alignment and appropriate implant placement. The patient recovered uneventfully from anaesthesia, began eating within 3 hours, and was discharged later the same day. Meloxicam and metoclopramide were continued for 5 days at previously detailed dosages. A course of oral enrofloxacin at 5 mg/kg twice daily for 5 days was also prescribed. Strict activity restriction was advised with large crate confinement and non-slip surfaces being recommended before follow up in 3 weeks.

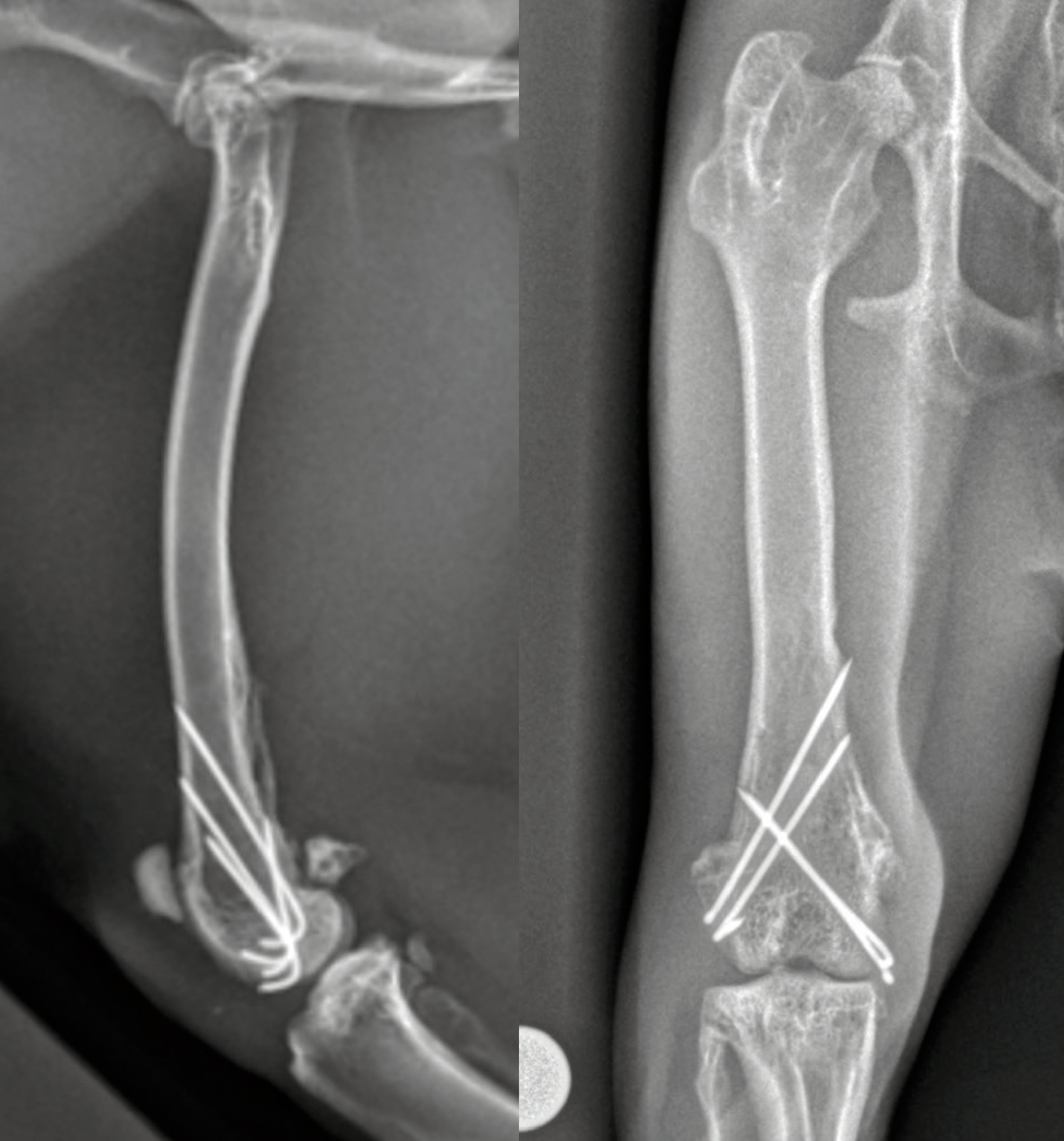
The owners reported intermittent mild right hindlimb lameness at home, 3 weeks after the procedure. The lameness was particularly evident after increased activity. All medications had been discontinued. A general physical examination was within normal expectations. The incision had healed with no associated swelling or erythema and adnexal structures were growing back in a patchy distribution. Gait assessment confirmed a mild, intermittent lameness affecting the right hindlimb. There was no pain response on manipulating the stifle or on palpating over the implants. Orthogonal radiographs of the right femur were performed. Sedation was achieved using ketamine 7 mg/kg subcuta- neously, dexmedetomidine 7 mcg/kg subcutaneously, methadone 0.5 mg/kg intramuscularly, and alfaxalone 2 mg/kg intramuscularly. Radiographs (Figure 3) showed that the alignment, apposition and implant placement were unchanged. There was evidence of appropriate osseous activity across the fracture site and a mineralised reaction at the attachment of the adductor magnus muscle noted on the mediolateral view. An increase in activity was advised with access to the house being permitted while the patient was being supervised.

The rabbit was assessed 6 weeks postoperatively. The right hindlimb lameness had improved, although there was still occasional reduced weightbearing through it, evidenced by reduced splaying of the digits. The remainder of the orthopaedic examination was the same as that conducted at the 3-week examination. Radiographs were not performed at this follow up because of the positive clinical progress and a desire to avoid potential sedation risks. A return to normal activity levels was advised at this time.
At 12 weeks postoperative follow up, the rabbit had resumed normal activities and the right hindlimb lameness had resolved. The remainder of the orthopaedic examination remained the same. Sedation was achieved using the same protocol as detailed for the visit at 3 weeks postoperatively and orthogonal views of the right femur were taken (Figure 4). An identically positioned craniocaudal view of the left femur was also taken to allow comparison of femoral length. Radiographs of the right femur revealed that alignment, apposition and implant placement had been maintained, and no evidence of implant-associated complications. Clinical union of the fracture had been achieved. There was no difference in length between the right and left femora and all femoral physes were closed.
Remote follow-up with the owners 7 months after the operation revealed that the rabbit had experienced no adverse sequelae following fracture repair. No lameness or pain was perceived by the owners and the rabbit had returned to a completely normal lifestyle and activity level.
Discussion
There is a paucity of knowledge on the treatment of and prognosis for fractures in rabbits, although the knowledge has increased in recent years. This report, detailing satisfactory medium-term follow up after surgical stabilisation of a Salter-Harris type I distal femoral fracture, contributes to this sparse evidence base.
Fractures of the hindlimbs occur more commonly than fractures of the forelimbs in rabbits (Sasai et al, 2015). Rabbits carry 70% of their bodyweight on their hindlimbs (Massie et al, 2019) and are less able than dogs and cats to shift weight away from a hindlimb following stabilisation of a fracture. Additionally, rabbits are very active, have a strong startle response and are highly sensitive to perceived threats (Jenkins, 2001), which makes it difficult to successfully restrict activity levels in the postoperative period. Accordingly, fracture repair must be sufficiently stable to allow weightbearing in the immediate postoperative period, and enable animals to return to all normal behaviours (Sasai et al, 2018).
In this case, achieving anatomical reduction was a key component of attaining adequate stability of the fracture. Anatomical reduction of distal femoral physeal fractures will provide some rotational stability as a result of the four pegs extending from the distal metaphysis, which align with four corresponding recessed regions in the distal epiphysis. Under-reduction, varus or valgus malalign-ment were all actively avoided because these are associated with greater risks of implant migration and failure (Hardie and Chambers, 1984; Schrader, 1994; Piermattei et al, 2006). Additionally, intraoperative fluoroscopy was used to confirm both reduction and appropriate implant placement, including that the cross-over point of the pins was located above the fracture line (Boekhout-Ta et al, 2017). This is critical in providing sufficient stability and the best prognosis. An alternative method of stabilisation for this case would have been the use of Rush pins, as this method is considered equally effective at neutralising forces across the physis when compared to cross-pinning (Sukhiani and Holmberg, 1997).
Rabbit bone is extremely brittle (Massie et al, 2019). Iatro-genic fractures during implant placement (Barron et al, 2010) and failure of the bone implant interface (Barron et al, 2010; Hak et al, 2011), leading to peri-implant failure and loss of fixation, are commonly encountered. In order to reduce the risk of such complications following fracture stabilisation, it has long been recommended that any iatrogenically created bone defect should be limited to a size resulting in a loss of no more than half the bone strength (Edgerton et al, 1990). In sheep, this led to the recommendation that the maximum size of any created bone defect should be 33% of the bone diameter (Edgerton et al, 1990), and this practice has become the standard widely used in orthopaedic surgery for companion animals. A study has shown that, likely because of structural differences in bone between species, in rabbits the maximum size of a created defect should be no more than 15% of bone diameter in order to maintain satisfactory bone strength (Massie et al, 2019).
In order to accommodate the unique and brittle biomechanics of rabbit bone, the surgical technique was adapted in this case. The use of fragment-holding forceps on the small distal fragment was avoided in order to reduce the risk of iatrogenic fracture. Additionally, to maintain adequate bone strength, small 0.7 mm Kirschner wires were selected; a size that represented approximately 10% of the bone diameter in this case.
The use of enrofloxacin for both perioperative and postoperative antibiosis in this case raises some concern regarding the potential for development of cartilage abnormalities. Non-inflammatory, erosive arthropathies can be observed in growing animals treated with fluoroquinolones (Brown, 1996) with these lesions being preferentially located at weightbearing joints (Neu, 1988). This has led to recommendations to avoid these medications in skeletally immature dogs, particularly those of large breeds (Boothe, 2012). However, articular cartilage vesicles generally form after a single large dose, or several moderately large doses of enrofloxacin (Brown, 1996).
In rabbits, enrofloxacin doses of 10-20 mg/kg are frequently used (Hedley, 2018). The use of enrofloxacin at 20 mg/kg once daily in 6-month-old Soviet Chinchilla rabbits has been reported experimentally for up to 21 days and, although this was not a primary outcome measure of this study, joint lesions or lameness were not detected (Khan and Rampal, 2013). The lower doses of 5 mg/kg, as used in this case, were considered low risk and neither follow-up orthopaedic examinations nor radiographs revealed evidence of joint disease. However, alternative options for perioperative antibiosis could have been considered, including trimethoprim-sulfonamides.
In canine and feline patients, the prognosis for limb function following repair of Salter-Harris type I and type II fractures is good to excellent when the appropriate pinning techniques are used (Johnson et al, 1994; Piermattei et al, 2006) and the satisfactory outcome in this case indicates that a similarly good prognosis is achievable in rabbits.
Distal femoral physeal fractures frequently result in premature physeal closure, as is the case for all physeal injuries (Brinker, 1974; Parker and Bloomberg, 1984; Culvenor et al, 1996). This has been observed in up to 83% of cases and is thought to result from the inciting trauma rather than surgical stabilisation techniques (Berg et al, 1984; Parker and Bloomberg, 1984; Johnson et al, 1994; Simpson and Lewis, 2002). The clinical impact that symmetric physeal closure will have on an individual animal will depend on age and how much more they could potentially grow. Rabbits reach sexual maturity at roughly 6 months, earlier than many other domestic species, and are considered skeletally mature at this time (Gilsanz et al, 1988). The physes remained open in the case reported here, but at 5 months of age this rabbit was almost skeletally mature and was considered at low risk of growth deformity. This was confirmed by the right and left femora being the same length on the final radiographs, taken 12 weeks postoperatively.
Conclusions
It is often stated that the surgical methods used for fracture stabilisation in dogs and cats are not necessarily appropriate for rabbits, yet the satisfactory medium-term outcomes reported here following cross-pin placement indicate that this might be an appropriate method of repairing distal femoral Salter-Harris type I fractures in this species. Consideration of the unique biomechanics of rabbit bone should play a key role in the planning of any fracture stabilisation.
Key points
- The majority of fractures sustained in rabbits are traumatic and associated with being trapped in cages, or being stepped on or dropped by owners.
- Rabbits bear 70% of their bodyweight through their hindlimbs and are less able to shift weight away from an injured limb than dogs and cats. Fracture fixation must be sufficiently stable to allow weightbearing in the immediate postoperative period.
- Rabbit bone is extremely brittle and any surgical technique must be adapted to account for this.
- The maximum size of a created defect in rabbit bone should be limited to 15% of the bone diameter.
- Rabbits are considered skeletally mature at 6 months of age. This should be taken into account when considering prognosis after a physeal fracture.
- Cross-pinning of Salter-Harris type I distal femoral physeal fractures warrants consideration and appears to offer a favourable prognosis.


